Key Results & Achievements
Butantan Phase III Trial
By July 2019, the Butantan Phase III trial had completed 99% of the recruitment of 17,000 subjects aged 2 to 59 in 16 geographic locations across Brazil to test the efficacy of a live attenuated tetravalent dengue vaccine. In the final 12 months, the focus was on recruiting younger children.
In parallel, evaluations were conducted to identify the most sensitive and specific assay for Zika seroprevalence studies. Traditionally, EuroImmune`s dengue IgG assay is used, but its high cross-reactivity with dengue is well documented. Instead, a new assay which is Brazil-based and developed by Advagen was identified. Well-characterized samples and control panels were obtained, and it was found that Advagen`s assay was superior. The Zika seroprevalence study is now being conducted using Advagen`s assay.
Birth Defect Surveillance
The Latin American Network of Congenital Malformations (Red Latino Americana de Malformaciones Congénitas: ReLAMC) was established in 2017 by Associação Técnico-Científica Estudo Colaborativo Latino Americano de Malformações Congênitas (ECLAMC). From 2017 to 2019 ReLAMC covered more than nine million births examined by 12 Latin American Congenital Anomaly registries.
A protocol for the surveillance of microcephaly and other congenital anomalies associated with maternal infections was presented to 16 Latin American Congenital Anomaly registries in Buenos Aires during the Annual ReLAMC meeting in November 2017. Nine registries have contributed data, the data have been analysed and a draft of a paper for submission to a peer review journal has been completed. Two protocols for surveillance of spina bifida and congenital anomaly in adolescent mothers were approved in Caxias do Sul, Brazil, during the last meeting in November 2019.
A paper on the use of infectious disease surveillance reports to PAHO/WHO to monitor the Zika virus epidemic in Latin America and the Caribbean, focusing on congenital zika syndrome, from 2015-2017 has been submitted for publication.
The Global Birth Defects website was launched. This website offers a comprehensive, up-to-date inventory of available resources and associated tools for congenital anomaly surveillance together with research that can be adapted to local needs and used to determine appropriate public health interventionsThe continuation of the committee should ensure that these efforts will be sustained well beyond the end of the ZikaPLAN project.
An App for the Description and Coding of Birth Defects at birth was developed. The App includes diagrams and photos of birth defects, ICD10 codes and definitions of 92 (85 major and 7 minor) externally visible anomalies and 9 syndromes (including Congenital Zika Syndrome) and will fill a gap in capacity for the accurate description and coding of birth defects in low resource environments. There are two versions: Basic and Surveillance. The Basic Version which does not allow recording of data was reviewed by experts, and show-cased and launched in October 2019. It is now available in Google and Apple Playstores and has been downloaded in over 39 countries. The Surveillance Version which allows recording of a small amount of data for diagnostic purposes is currently undergoing field testing in Africa and South America with anticipated launch in early 2021. th a physician.
International Traveler Surveillance and Study
In parallel with the decline in the incidence of Zika infections globally, cases in travelers have dropped dramatically. The working group continued to recruit international travelers bound for Brazil and to equip them with credit-card-size mosquito traps to collect mosquito-saliva in order to assess the arbovirom in the local mosquito population along the travelers’ routes.
In a second study, based in Barcelona, international travelers were equipped with a smartphone app., which assesses the incidence of arbovirus-compatible symptoms in international travelers and links the travelers in real-time with a physician.
In December 2018, the International Committee for Congenital Anomaly Surveillance Tools (comprising members from Europe, Latin America, Africa, Asia and the USA) was launched.
International Committee for Birth Defect Surveillance Tools
- Prof Helen Dolk (ZikaPLAN Co-investigator, Chair), Ulster University
- Prof Ieda Orioli (ZikaPLAN Co-investigator), representing ECLAMC/RELAMC
- Prof Ingeborg Barisic, Children’s University Hospital Zagreb, representing EUROCAT
- Dr Linda Barlow, The Makerere University-John Hopkins University Research Collaboration (MUHJU) Kampala
- Prof Lorenzo Botto, University of Utah, representing ICBDMSR
- Dr Ester Garne, Lillebaelt Hospital Denmark, representing EUROCAT
- Dr Pilar Guatibonza, representing ECLAMC
- Dr Christine Halleux, WHO-TDR, Geneva (Corrine Merle from August 2020)
- Dr. Cindy Moore, National Center on Birth Defects and Developmental Disabilities at the Centers for Disease Control and Prevention (CDC) Atlanta, USA.
- Prof Lewis Holmes, Massachusetts General Hospital Boston, representing WHO-TDR
- Dr Neena Raina WHO, Regional Office for South East Asia (WHO SEARO), New Delhi, India
- Ms Diana Valencia, Centres for Disease Control Atlanta, representing CDC
Participating Organisations
Group leader: Prof. Annelies Wilder-Smith, Umeå University
- London School of Hygiene & Tropical Medicine
- Queen Mary University of London
- Ulster University
- Fundação de Apoio à Universidade de São Paulo
- Instituto Butantan
- Associação Técnica–Científica de Estudo Colaborativo Latino Americano de Malformações Congênitas
- Schweizerisches Tropen- und Public Health-Institut
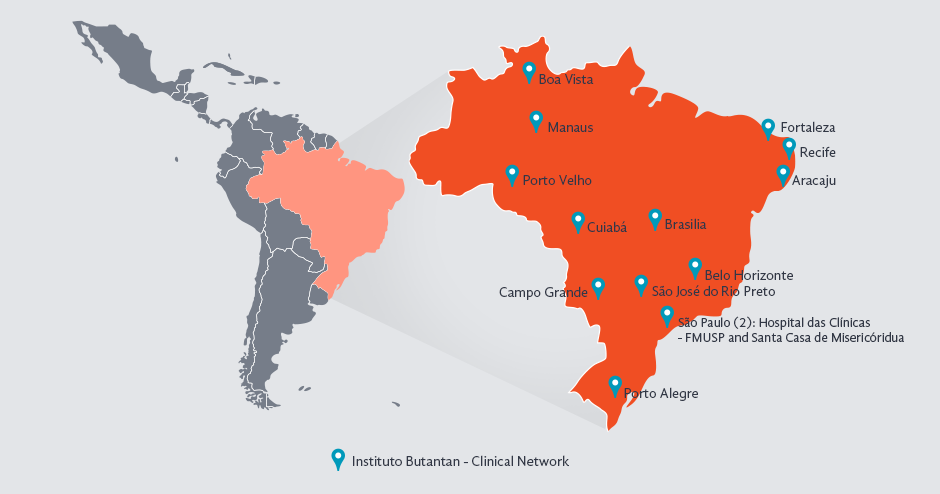
Prospective cohort study of 17,000 subjects aged 2-59 in 14 clinical sites in Brazil (Aracajú, Belo Horizonte, Boa Vista, Brasília, Campo Grande, Cuiabá, Fortaleza, Mananus, São José do Rio Preto, São Paulo (2): Hospital das Clínicas – FMUSP and Santa Casa de Misericóridua, Porto Alegre, Porto Velho and Recife)