July 3, 2018
NEURO-ZIKA pathogenesis work in ZikaPLAN is differentiating autoimmune versus direct neuropathic phenomena of ZIKV to impact both clinical care and basic understanding of disease.
 Some members of the Glasgow Zika Team photographed following a lab meeting at the Centre for Virus Research. Left to Right: Verena Schultz, Stephanie Cumberworth, Hugh Willison, Julia Edgar, Susan Barnett, Madeleine Cunningham, Alain Kohl.
Some members of the Glasgow Zika Team photographed following a lab meeting at the Centre for Virus Research. Left to Right: Verena Schultz, Stephanie Cumberworth, Hugh Willison, Julia Edgar, Susan Barnett, Madeleine Cunningham, Alain Kohl.
ZikaPLAN has a NEURO-ZIKA working group focused on the neuropathogenic features of Zika virus infection. As the distinction between Zika virus infection and autoimmunity greatly impacts both clinical care and basic understanding of disease, its investigation is very important. At University of Glasgow, a strong and highly functional collaboration has been formed between researchers within the Centre for Virus Research (CVR), (group leader, Alain Kohl, also a member of ZIKAlliance), and members of the Neuroscience group within the Institute of Infection, Inflammation and Immunity (3I), (group leaders, Hugh Willison, Susan Barnett, Julia Edgar, Chris Linington) who lead the ZikaPLAN program in Glasgow.
This close collaboration has created an exciting and vibrant research program involving many staff members locally and, further afield, extensive collaborative networks have been forged throughout Europe and Latin America. Importantly, this has shown that cross-consortium alliances can be built and links with other clinical centers in Latin America can be created. A vital contribution to this activity has been the additional funding secured from the Medical Research Council (MRC) and the Wellcome Trust.
Zika virus infection is linked to a wide range of seemingly distinct and severe neurological phenotypes affecting both the peripheral and central nervous systems (PNS and CNS). These include neuro-complications arising from direct infection of adult humans (e.g. viral encephalitis) and developing foetuses (e.g. microcephaly), and complications arising from autoimmune responses to neural tissues induced by Zika infection (e.g. Guillain-Barre syndrome (GBS)). In order to investigate these wholly unexplored pathways, a set of basic principles underlying the neuroimmune interactions in Zika infection is first being established.
The first task included determining which neural cell types are infected with Zika virus and how does tropism differ in the central and peripheral nervous systems. This knowledge would enhance understanding of the relationship between Zika virus infection and neural phenotypes. The work performed has revealed variation in neural susceptibility of cell types to viral infection which has major implications for the health of the developing brain. In particular, as well as seeing extensive astrocyte and nerve cell infection, demonstrating that oligodendrocytes can be infected has implications for the possible effects of childhood infection on the postnatal developing brain.
Zika virus tropism in the PNS and CNS has now been performed in vitro and these data are guiding the progress to in vivo work. Different viral isolates show different tropism for nervous system cells. Infection of neurons clearly leads to a specific axonal phenotype without inducing neuronal cell death which will be investigated in trafficking studies once reagents become available.
The comparative analysis of PNS and CNS infection has demonstrated important mechanistic insights. It is necessary to discover whether Zika virus causes GBS through direct neurotropism/neuroinfection, or through post-infectious autoimmunity. If the latter, can the antigens involved that will allow development of diagnostic testing be identified? To this end, a substantial biobank of Zika serum samples from colalborators throughout Latin America has been built.
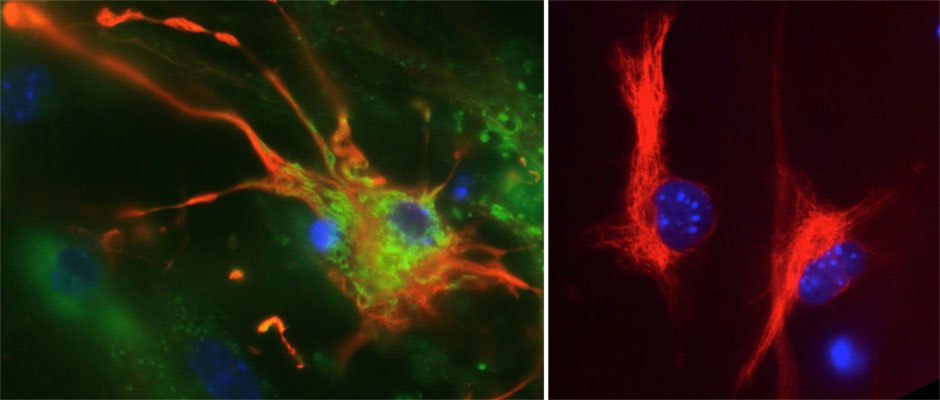 A brain astrocyte cell (colored red) infected with Zika virus, shown in green (left panel), alongside an uninfected astrocyte (right panel)
A brain astrocyte cell (colored red) infected with Zika virus, shown in green (left panel), alongside an uninfected astrocyte (right panel)
Studies to investigate whether humoral factors from Zika-GBS patient and control sera are pathogenic to PNS and CNS cells in vitro, are well underway. Sera from Zika-GBS cases and controls are being screened for antigen targets known to be associated with conventional GBS using sensitive immunoassays: to date, these have not been found, indicating the Zika-GBS may have a unique autoimmune signature. This is currently being investigated using different in vitro culture systems.
The work performed so far has been presented at a wide range of local, national and international seminars and conferences by the principal investigators involved in the studies and by junior staff and PhD students. The enthusiasm for the project amongst all involved in the work has been a great strength in driving forward discoveries at a fast pace and further progress will be welcomed over the coming years.